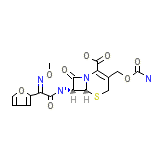
Cefditoren




Cefditoren Brand names, Cefditoren Analogs
- Ancef
- Biofuroksym
- Ceclor
- Ceclor CD
- Cedax
- Cefditoren
- Cefizox
- Cefobid
- Cefotan
- Ceftin
- Cefurax
- Cefuril
- Cefuroxim
- Cefuroxime [USAN:BAN:INN]
- Cefuroximo [INN-Spanish]
- Cefuroximum [INN-Latin]
- Cefzil
- Cepazine
- Cephuroxime
- Ceptaz
- Duricef
- Elobact
- Fortaz
- Keflex
- Keftab
- Kefurox
- Kefzol
- Kerurox
- Mandol
- Maxipime
- Mefoxin
- Monocid
- Omnicef
- Oraxim
- Rocephin
- Sharox
- Tazicef
- Vantin
- Velosef
- Zinacef
- Zinacef Danmark
- Zinat
- Zinnat
Cefditoren Brand Names Mixture
- No information avaliable
Cefditoren Chemical_Formula
Cefditoren RX_link
Cefditoren fda sheet
Cefditoren msds (material safety sheet)
Cefditoren Synthesis Reference
Cefditoren Molecular Weight
Cefditoren Melting Point
Cefditoren H2O Solubility
Cefditoren State
Cefditoren LogP
Cefditoren Dosage Forms
Cefditoren Indication
Cefditoren Pharmacology
Cefditoren Absorption
Cefditoren side effects and Toxicity
Cefditoren Patient Information
Patients should be counseled that antibacterial drugs including Cefuroxime for Injection USP and Dextrose Injection USP should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Cefuroxime for Injection USP and Dextrose Injection USP is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Cefuroxime for Injection USP and Dextrose Injection USP or other antibacterial drugs in the future.