Carmubris




Carmubris Brand names, Carmubris Analogs
Carmubris Brand Names Mixture
- No information avaliable
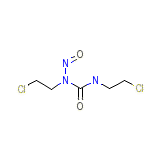
Carmubris Chemical_Formula
C5H9Cl2N3O2
Carmubris RX_link
http://www.rxlist.com/cgi/generic3/carmustine.htm
Carmubris fda sheet
Carmubris msds (material safety sheet)
Carmubris Synthesis Reference
Johnston TP et al. J Med Chem 122:669-681(1963).
Carmubris Molecular Weight
214.049 g/mol
Carmubris Melting Point
31 oC
Carmubris H2O Solubility
< 0.1 g/100 mL at 18 °C
Carmubris State
Solid
Carmubris LogP
1.256
Carmubris Dosage Forms
Powder for solution; Wafer
Carmubris Indication
For the treatment of brain tumors, multiple myeloma, Hodgkin's disease and Non-Hodgkin's lymphomas.
Carmubris Pharmacology
Carmustine is one of the nitrosoureas indicated as palliative therapy as a single agent or in established combination therapy with other approved chemotherapeutic agents in treatment of brain tumors, multiple myeloma, Hodgkin's disease, and non-Hodgkin's lymphomas. Although it is generally agreed that carmustine alkylates DNA and RNA, it is not cross resistant with other alkylators. As with other nitrosoureas, it may also inhibit several key enzymatic processes by carbamoylation of amino acids in proteins.
Carmubris Absorption
5 to 28% bioavailability
Carmubris side effects and Toxicity
The oral LD50s in rat and mouse are 20 mg/kg and 45 mg/kg, respectively. Side effects include leukopenia, thrombocytopenia, nausea. Toxic effects include pulmonary fibrosis (20-0%) and bone marrow toxicity.
Carmubris Patient Information
No information avaliable
Carmubris Organisms Affected
Humans and other mammals