CDTR-PI




CDTR-PI Brand names, CDTR-PI Analogs
CDTR-PI Brand Names Mixture
- No information avaliable
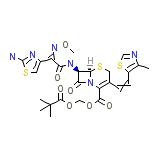
CDTR-PI Chemical_Formula
C25H28N6O7S3
CDTR-PI RX_link
http://www.rxlist.com/cgi/generic3/spectracef.htm
CDTR-PI fda sheet
CDTR-PI msds (material safety sheet)
CDTR-PI Synthesis Reference
No information avaliable
CDTR-PI Molecular Weight
620.724 g/mol
CDTR-PI Melting Point
127 - 129 oC
CDTR-PI H2O Solubility
Soluble at levels equal to < 0.1 mg/mL.
CDTR-PI State
Solid
CDTR-PI LogP
2.721
CDTR-PI Dosage Forms
Tablets (200mg) for oral administration.
CDTR-PI Indication
For the treatment of mild to moderate infections in adults and adolescents (12 years of age or older) which are caused by susceptible strains of microorganisms in acute bacterial exacerbation of chronic bronchitis, community-acquired pneumonia, pharyngitis/tonsillitis, and uncomplicated skin and skin-structure infections.
CDTR-PI Pharmacology
Cefditoren pivoxil is a prodrug which is hydrolyzed by esterases during absorption, and the drug is distributed in the circulating blood as active cefditoren. Cefditoren is a cephalosporin with antibacterial activity against gram-positive and gram-negative pathogens. Cefditoren is effective against Staphylococcus aureus (methicillin-susceptible strains, including b-lactamase-producing strains), penicillin-susceptible strains of Staphylococcus aureus and Streptococcus pneumoniae, Streptococcus pyogenes, Haemophilus influenzae (including b-lactamase-producing strains), Haemophilus parainfluenzae (including b-lactamase-producing strains), Moraxella catarrhalis (including b-lactamase-producing strains), Streptococcus agalactiae, Streptococcus Groups C and G, and Streptococcus, viridans group (penicillin-susceptible and -intermediate strains).
CDTR-PI Absorption
Following oral administration, cefditoren pivoxil is absorbed from the gastrointestinal tract and hydrolyzed to cefditoren by esterases. Under fasting conditions, the estimated absolute bioavailability of cefditoren pivoxil is approximately 14%. The absolute bioavailability of cefditoren pivoxil administered with a low fat meal (693 cal, 14 g fat, 122 g carb, 23 g protein) is 16.1 ± 3.0%.
CDTR-PI side effects and Toxicity
Information on cefditoren pivoxil overdosage in humans is not available. However, with other b-lactam antibiotics, adverse effects following overdosage have included nausea, vomiting, epigastric distress, diarrhea, and convulsions. In acute animal toxicity studies, cefditoren pivoxil when tested at the limit oral doses of 5100 mg/kg in rats and up to 2000 mg/kg in dogs did not exhibit any health effects of concern.
CDTR-PI Patient Information
No information avaliable
CDTR-PI Organisms Affected
Enteric bacteria and other eubacteria