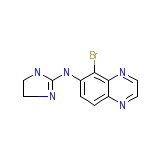
Brimonidine




Brimonidine Brand names, Brimonidine Analogs
Brimonidine Brand Names Mixture
- None
Brimonidine Chemical_Formula
C11H10BrN5
Brimonidine RX_link
http://www.rxlist.com/cgi/generic3/alphagan-p.htm
Brimonidine fda sheet
Brimonidine msds (material safety sheet)
Brimonidine Synthesis Reference
M. B. Thomas, M. T. Williams, Ger. Pat. 2,538,630 (1976 to Pfizer)
Brimonidine Molecular Weight
292.135 g/mol
Brimonidine Melting Point
207.5 oC
Brimonidine H2O Solubility
Soluble (1.5 mg/mL)
Brimonidine State
Solid
Brimonidine LogP
0.782
Brimonidine Dosage Forms
0.2% solution
Brimonidine Indication
For the lowering of intraocular pressure in patients with open-angle glaucoma or ocular hypertension.
Brimonidine Pharmacology
Brimonidine significantly lowers intraocular pressure with minimal effects on cardiovascular and pulmonary parameters. It lowers intraocular pressure by reducing aqueous humor production and increasing uveoscleral outflow.
Brimonidine Absorption
Minimal systemic absorption occurs after ocular insertion.
Brimonidine side effects and Toxicity
Oral LD50 is 50 mg/kg in mice and 100 mg/kg in rats.
Brimonidine Patient Information
PATIENT INFORMATION
Brimonidine tartrate is used for the treatment of open-angle glaucoma and ocular hypertension. Do not take this drug if you are currently taking MAO Inhibitors (e.g., Parnate). Inform your doctor if you are pregnant or nursing.
This product may stain soft contact lenses. Wait at least 15 minutes after instilling brimonidine to insert contact lenses.
May cause drowsiness or tiredness; use caution while driving or operating hazardous machinery. May cause dry mouth, blurring, stinging, or burning of the eyes; headaches, fatigue or drowsiness; allergic or itching of the eyes. Tell your doctor or pharmacist if these effects occur.
Brimonidine tartrate is used for the treatment of open-angle glaucoma and ocular hypertension. Do not take this drug if you are currently taking MAO Inhibitors (e.g., Parnate). Inform your doctor if you are pregnant or nursing.
This product may stain soft contact lenses. Wait at least 15 minutes after instilling brimonidine to insert contact lenses.
May cause drowsiness or tiredness; use caution while driving or operating hazardous machinery. May cause dry mouth, blurring, stinging, or burning of the eyes; headaches, fatigue or drowsiness; allergic or itching of the eyes. Tell your doctor or pharmacist if these effects occur.
Brimonidine Organisms Affected
Humans and other mammals