Basofortina




Basofortina Brand names, Basofortina Analogs
Basofortina Brand Names Mixture
- No information avaliable
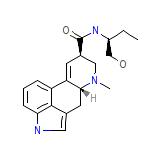
Basofortina Chemical_Formula
C20H25N3O2
Basofortina RX_link
http://www.rxlist.com/cgi/generic2/methylerg.htm
Basofortina fda sheet
Basofortina msds (material safety sheet)
Basofortina Synthesis Reference
A. Stoll et al., eidem, Helv. Chim. Acta. 26, 944 (1943)
Basofortina Molecular Weight
339.432 g/mol
Basofortina Melting Point
172 oC
Basofortina H2O Solubility
25 mg/mL
Basofortina State
Solid
Basofortina LogP
1.01
Basofortina Dosage Forms
Injection (intramuscular or intravenous); Tablet
Basofortina Indication
For the prevention and control of excessive bleeding following vaginal childbirth
Basofortina Pharmacology
Methylergonovine is a semisynthetic ergot alkaloid and a derivative of ergonovine and is used for the prevention and control of postpartum and post-abortion hemorrhage. In general, the effects of all the ergot alkaloids appear to results from their actions as partial agonists or antagonists at adrenergic, dopaminergic, and tryptaminergic receptors. The spectrum of effects depends on the agent, dosage, species, tissue, and experimental or physiological conditions. All of the alkaloids of ergot significantly increase the motor activity of the uterus. After small doses contractions are increased in force or frequency, or both, but are followed by a normal degree of relaxation. As the dose is increased, contractions become more forceful and prolonged, resting tonus is markedly increased, and sustained contracture can result.
Basofortina Absorption
Absorption is rapid after oral (60% bioavailability) and intramuscular (78% bioavailability) administration.
Basofortina side effects and Toxicity
Signs and symptoms of overexposure: hypertension, seizures, headache, hypotension, nausea, and vomiting.
Basofortina Patient Information
Basofortina Organisms Affected
Humans and other mammals