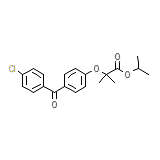
Antara




Antara Brand names, Antara Analogs
- Ankebin
- Antara
- Elasterate
- Elasterin
- FNF
- Fenobrate
- Fenofibrate [Ban:Inn]
- Fenofibrato [Inn-Spanish]
- Fenofibratum [Inn-Latin]
- Fenogal
- Fenotard
- Finofibrate
- Lipanthyl
- Lipantil
- Lipidex
- Lipidil
- Lipidil Micro
- Lipidil Supra
- Lipifen
- Lipirex
- Lipoclar
- Lipofene
- Liposit
- Lipsin
- Lofibra
- Luxacor
- Nolipax
- Procetofen
- Proctofene
- Protolipan
- Secalip
- Sedufen
- Tricor
- Triglide
Antara Brand Names Mixture
- No information avaliable
Antara Chemical_Formula
C20H21ClO4
Antara RX_link
http://www.rxlist.com/cgi/generic3/fenofibrate.htm
Antara fda sheet
Antara msds (material safety sheet)
Antara Synthesis Reference
No information avaliable
Antara Molecular Weight
360.831 g/mol
Antara Melting Point
80.5 °C
Antara H2O Solubility
0.25mg/ml at 25 °C
Antara State
Solid
Antara LogP
5.575
Antara Dosage Forms
Capsule; Tablet
Antara Indication
For use as adjunctive therapy to diet to reduce elevated LDL-C, Total-C,Triglycerides and Apo B, and to increase HDL-C in adult patients with primary hypercholesterolemia or mixed dyslipidemia (Fredrickson Types IIa and IIb)
Antara Pharmacology
Fenofibrate is a lipid regulating agent indicated as adjunctive therapy to diet to reduce elevated LDL-C, Total-C,Triglycerides and Apo B, and to increase HDL-C in adult patients with primary hypercholesterolemia or mixed dyslipidemia (Fredrickson Types IIa and IIb). Fenofibrate is also indicated as adjunctive therapy to diet for treatment of adult patients with hypertriglyceridemia (Fredrickson Types IV and V hyperlipidemia). Fenofibric acid, the active metabolite of Fenofibrate, produces reductions in total cholesterol, LDL cholesterol, apolipoprotein B, total triglycerides and triglyceride rich lipoprotein (VLDL) in treated patients. In addition, treatment with fenofibrate results in increases in high density lipoprotein (HDL) and apoproteins apoAI and apoAII.
Antara Absorption
Fenofibrate is well absorbed from the gastrointestinal tract. After absorption, fenofibrate is mainly excreted in the urine in the form of metabolites, primarily fenofibric acid and fenofibric acid glucuronide
Antara side effects and Toxicity
LD50=1600 mg/kg (Oral, in mice); Investigated as a teratogen and reproductive hazard.
Antara Patient Information
No information avaliable
Antara Organisms Affected
Humans and other mammals