Allegra




Allegra Brand names, Allegra Analogs
Allegra Brand Names Mixture
- Allegra-D (Fexofenadine hydrochloride + Pseudoephedrine hydrochloride)
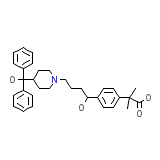
Allegra Chemical_Formula
Allegra RX_link
Allegra fda sheet
Allegra msds (material safety sheet)
Allegra Synthesis Reference
Allegra Molecular Weight
Allegra Melting Point
Allegra H2O Solubility
Allegra State
Allegra LogP
Allegra Dosage Forms
Allegra Indication
Allegra Pharmacology
Allegra Absorption
Allegra side effects and Toxicity
Allegra Patient Information
Patients taking ALLEGRA tablets should receive the following information:
ALLEGRA tablets are prescribed for the relief of symptoms of seasonal allergic rhinitis or for the relief of symptoms of chronic idiopathic urticaria (hives). Patients should be instructed to take ALLEGRA only as prescribed. Do not exceed the recommended dose. If any untoward effects occur while taking ALLEGRA, discontinue use and consult the doctor.
The product should not be used by patients who are hypersensitive to it or to any of its ingredients.
Patients should be told that this product should be used in pregnancy or lactation only if the potential benefit justifies the potential risk to the fetus or nursing infant.
Patients should be advised to take the tablet with water. Patients should also be advised to store the medication in a tightly closed container in a cool, dry place, away from children.