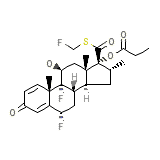
Advair Diskus 100/50




Advair Diskus 100/50 Brand names, Advair Diskus 100/50 Analogs
Advair Diskus 100/50 Brand Names Mixture
- No information avaliable
Advair Diskus 100/50 Chemical_Formula
C25H31F3O5S
Advair Diskus 100/50 RX_link
http://www.rxlist.com/cgi/generic2/cutivate.htm
Advair Diskus 100/50 fda sheet
Advair Diskus 100/50 msds (material safety sheet)
Advair Diskus 100/50 Synthesis Reference
G. H. Phillipps, et. Al., U.S. Pat. 4,335,121 (1982)
Advair Diskus 100/50 Molecular Weight
500.572 g/mol
Advair Diskus 100/50 Melting Point
272-273 oC
Advair Diskus 100/50 H2O Solubility
0.51 mg/L (insoluble)
Advair Diskus 100/50 State
Solid
Advair Diskus 100/50 LogP
4.068
Advair Diskus 100/50 Dosage Forms
Metered-dose (aerosol)
Advair Diskus 100/50 Indication
For the maintenance treatment of asthma as prophylactic therapy and for patients requiring oral corticosteroid therapy for asthma.
Advair Diskus 100/50 Pharmacology
Fluticasone propionate, a medium-potency synthetic corticosteroid, is used topically to relieve inflammatory and pruritic symptoms of dermatoses and psoriasis, intranasally to manage symptoms of allergic and non-allergic rhinitis, and orally for the treatment of asthma.
Advair Diskus 100/50 Absorption
The extent of percutaneous absorption of topical corticosteroids is determined by many factors, including the vehicle and the integrity of the epidermal barrier.
Advair Diskus 100/50 side effects and Toxicity
No information avaliable
Advair Diskus 100/50 Patient Information
No information avaliable
Advair Diskus 100/50 Organisms Affected
Humans and other mammals