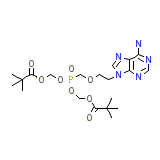
Adefovir




Adefovir Brand names, Adefovir Analogs
Adefovir Brand Names Mixture
- No information avaliable
Adefovir Chemical_Formula
C20H32N5O8P
Adefovir RX_link
http://www.rxlist.com/cgi/generic3/hepsera.htm
Adefovir fda sheet
Adefovir msds (material safety sheet)
Adefovir Synthesis Reference
No information avaliable
Adefovir Molecular Weight
501.471 g/mol
Adefovir Melting Point
No information avaliable
Adefovir H2O Solubility
19 mg/mL at pH 2.0 and 0.4 mg/mL at pH 7.2.
Adefovir State
Solid
Adefovir LogP
3.649
Adefovir Dosage Forms
Tablet (10 mg)
Adefovir Indication
For the treatment of chronic hepatitis B in adults with evidence of active viral replication and either evidence of persistent elevations in serum aminotransferases (ALT or AST) or histologically active disease.
Adefovir Pharmacology
Adefovir dipivoxil a diester prodrug of adefovir. Adefovir is an acyclic nucleotide analog with activity against human hepatitis B virus (HBV). The concentration of adefovir that inhibited 50% of viral DNA synthesis (IC50) in vitro ranged from 0.2 to 2.5 μM in HBV transfected human hepatoma cell lines. The combination of adefovir with lamivudine showed additive anti-HBV activity.
Adefovir Absorption
Approximate oral bioavailability is 59%.
Adefovir side effects and Toxicity
Renal tubular nephropathy characterized by histological alterations and/or increases in BUN and serum creatinine was the primary dose-limiting toxicity associated with administration of adefovir dipivoxil in animals. Nephrotoxicity was observed in animals at systemic exposures approximately 3–10 times higher than those in humans at the recommended therapeutic dose of 10 mg/day.
Adefovir Patient Information
Adefovir Organisms Affected
Hepatitis B virus