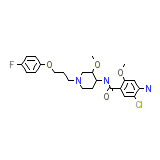
Acenalin




Acenalin Brand names, Acenalin Analogs
Acenalin Brand Names Mixture
- No information avaliable
Acenalin Chemical_Formula
C23H29ClFN3O4
Acenalin RX_link
http://www.rxlist.com/cgi/generic/cisap.htm
Acenalin fda sheet
Acenalin msds (material safety sheet)
Acenalin Synthesis Reference
No information avaliable
Acenalin Molecular Weight
465.945 g/mol
Acenalin Melting Point
110 oC
Acenalin H2O Solubility
2.71 mg/L
Acenalin State
Solid
Acenalin LogP
3.802
Acenalin Dosage Forms
Suspension; Tablet
Acenalin Indication
For the symptomatic treatment of adult patients with nocturnal heartburn due to gastroesophageal reflux disease.
Acenalin Pharmacology
Cisapride is a parasympathomimetic which acts as a serotonin 5-HT4 agonist. Stimulation of the serotonin receptors increases acetylcholine release in the enteric nervous system. Cisapride stimulates motility of the upper gastrointestinal tract without stimulating gastric, biliary, or pancreatic secretions. Cisapride increases the tone and amplitude of gastric (especially antral) contractions, relaxes the pyloric sphincter and the duodenal bulb, and increases peristalsis of the duodenum and jejunum resulting in accelerated gastric emptying and intestinal transit. It increases the resting tone of the lower esophageal sphincter. It has little, if any, effect on the motility of the colon or gallbladder. Cisapride does not induce muscarinic or nicotinic receptor stimulation, nor does it inhibit acetylcholinesterase activity.
Acenalin Absorption
Cisapride is rapidly absorbed after oral administration, with an absolute bioavailability of 35-40%.
Acenalin side effects and Toxicity
No information avaliable
Acenalin Patient Information
No information avaliable
Acenalin Organisms Affected
Humans and other mammals