ATV




ATV Brand names, ATV Analogs
ATV Brand Names Mixture
- No information avaliable
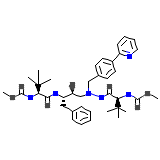
ATV Chemical_Formula
C38H52N6O7
ATV RX_link
http://www.rxlist.com/cgi/generic3/reyataz.htm
ATV fda sheet
ATV msds (material safety sheet)
ATV Synthesis Reference
No information avaliable
ATV Molecular Weight
704.856 g/mol
ATV Melting Point
No information avaliable
ATV H2O Solubility
Free base slightly soluble (4-5 mg/mL)
ATV State
Solid
ATV LogP
4.25
ATV Dosage Forms
Capsule (100 mg, 150 mg, or 200 mg)
ATV Indication
For use, in combination with other antiretroviral agents, in the treatment of HIV-1 infection.
ATV Pharmacology
Atazanavir (ATV) is an azapeptide HIV-1 protease inhibitor (PI). It is a protease inhibitor with activity against Human Immunodeficiency Virus Type 1 (HIV-1). Protease inhibitors block the part of HIV called protease. HIV-1 protease is an enzyme required for the proteolytic cleavage of the viral polyprotein precursors into the individual functional proteins found in infectious HIV-1. Atazanavir binds to the protease active site and inhibits the activity of the enzyme. This inhibition prevents cleavage of the viral polyproteins resulting in the formation of immature non-infectious viral particles. Protease inhibitors are almost always used in combination with at least two other anti-HIV drugs.
ATV Absorption
Atazanavir is rapidly absorbed with a Tmax of approximately 2.5 hours. Administration of atazanavir with food enhances bioavailability and reduces pharmacokinetic variability
ATV side effects and Toxicity
No information avaliable
ATV Patient Information
ATV Organisms Affected
Human immunodeficiency virus