AG-EE 388 ZW




AG-EE 388 ZW Brand names, AG-EE 388 ZW Analogs
AG-EE 388 ZW Brand Names Mixture
- No information avaliable
AG-EE 388 ZW Chemical_Formula
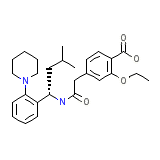
C27H36N2O4
AG-EE 388 ZW RX_link
http://www.rxlist.com/cgi/generic/prandin.htm
AG-EE 388 ZW fda sheet
AG-EE 388 ZW msds (material safety sheet)
AG-EE 388 ZW Synthesis Reference
W. Grell et al., PCT Int. pat. Appl. WO 93 00,337
AG-EE 388 ZW Molecular Weight
452.586 g/mol
AG-EE 388 ZW Melting Point
130-131 oC
AG-EE 388 ZW H2O Solubility
No information avaliable
AG-EE 388 ZW State
Solid
AG-EE 388 ZW LogP
5.798
AG-EE 388 ZW Dosage Forms
Tablet
AG-EE 388 ZW Indication
For the treatment of Type II diabetes mellitus.
AG-EE 388 ZW Pharmacology
Repaglinide is an oral blood glucose-lowering drug of the meglitinide class used in the management of type 2 diabetes mellitus (also known as non-insulin dependent diabetes mellitus or NIDDM). Repaglinide lowers blood glucose levels by stimulating the release of insulin from the pancreas. This action is dependent upon functioning beta cells in the pancreatic islets. Insulin release is glucose-dependent and diminishes at low glucose concentrations.
AG-EE 388 ZW Absorption
Rapid (bioavailability is 56%)
AG-EE 388 ZW side effects and Toxicity
LD50 >1 g/kg (rat) (W. Grell)
AG-EE 388 ZW Patient Information
PATIENT INFORMATION
Patients should be informed of the potential risks and advantages of Repaglinide (PRANDIN) and of alternative modes of therapy. They should also be informed about the importance of adherence to dietary instructions, of a regular exercise program, and of regular testing of blood glucose and HbA1c. The risks of hypoglycemia, its symptoms and treatment, and conditions that predispose to its development and concomitant administration of other glucose-lowering drugs should be explained to patients and responsible family members. Primary and secondary failure should also be explained.
Patients should be instructed to take Repaglinide before meals (2, 3, or 4 times a day preprandially). Doses are usually taken within 15 minutes of the meal but time may vary from immediately preceding the meal to as long as 30 minutes before the meal. Patients who skip a meal (or add an extra meal) should be instructed to skip (or add) a dose for that meal.
Patients should be informed of the potential risks and advantages of Repaglinide (PRANDIN) and of alternative modes of therapy. They should also be informed about the importance of adherence to dietary instructions, of a regular exercise program, and of regular testing of blood glucose and HbA1c. The risks of hypoglycemia, its symptoms and treatment, and conditions that predispose to its development and concomitant administration of other glucose-lowering drugs should be explained to patients and responsible family members. Primary and secondary failure should also be explained.
Patients should be instructed to take Repaglinide before meals (2, 3, or 4 times a day preprandially). Doses are usually taken within 15 minutes of the meal but time may vary from immediately preceding the meal to as long as 30 minutes before the meal. Patients who skip a meal (or add an extra meal) should be instructed to skip (or add) a dose for that meal.
AG-EE 388 ZW Organisms Affected
Humans and other mammals