ABC




ABC Brand names, ABC Analogs
ABC Brand Names Mixture
- Kivexa (Abacavir Sulfate + Lamivudine)
- Trizivir (Abacavir Sulfate + Lamivudine + Zidovudine)
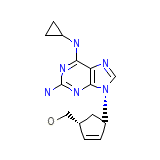
ABC Chemical_Formula
ABC RX_link
ABC fda sheet
ABC msds (material safety sheet)
ABC Synthesis Reference
ABC Molecular Weight
ABC Melting Point
ABC H2O Solubility
ABC State
ABC LogP
ABC Dosage Forms
ABC Indication
ABC Pharmacology
ABC Absorption
ABC side effects and Toxicity
ABC Patient Information
Hypersensitivity Reaction: Inform patients:
- that a Medication Guide and Warning Card summarizing the symptoms of the abacavir hypersensitivity reaction and other product information will be dispensed by the pharmacist with each new prescription and refill of ZIAGEN, and encourage the patient to read the Medication Guide and Warning Card every time to obtain any new information that may be present about ZIAGEN. (The complete text of the Medication Guide is reprinted at the end of this document.)
- to carry the Warning Card with them.
- how to identify a hypersensitivity reaction.
- that if they develop symptoms consistent with a hypersensitivity reaction to discontinue treatment with ZIAGEN and seek medical evaluation immediately.
- that a hypersensitivity reaction can worsen and lead to hospitalization or death if ZIAGEN is not immediately discontinued.
- that in one study, more severe hypersensitivity reactions were seen when ZIAGEN was dosed 600 mg once daily.
- to not restart ZIAGEN or any other abacavir-containing product following a hypersensitivity reaction because more severe symptoms can occur within hours and may include life-threatening hypotension and death.
- that a hypersensitivity reaction is usually reversible if it is detected promptly and ZIAGEN is stopped right away.
- that if they have interrupted ZIAGEN for reasons other than symptoms of hypersensitivity (for example, those who have an interruption in drug supply), a serious or fatal hypersensitivity reaction may occur with reintroduction of abacavir.
- to not restart ZIAGEN or any other abacavir-containing product without medical consultation and that restarting abacavir needs to be undertaken only if medical care can be readily accessed by the patient or others.
General: Inform patients that some HIV medicines, including ZIAGEN, can cause a rare, but serious condition called lactic acidosis with liver enlargement (hepatomegaly).
ZIAGEN is not a cure for HIV infection and patients may continue to experience illnesses associated with HIV infection, including opportunistic infections. Patients should remain under the care of a physician when using ZIAGEN. Advise patients that the use of ZIAGEN has not been shown to reduce the risk of transmission of HIV to others through sexual contact or blood contamination.
Inform patients that redistribution or accumulation of body fat may occur in patients receiving antiretroviral therapy and that the cause and long-term health effects of these conditions are not known at this time.
ZIAGEN Tablets and Oral Solution are for oral ingestion only. Patients should be advised of the importance of taking ZIAGEN exactly as it is prescribed.
MEDICATION GUIDE
ZIAGEN® (z-EYE-uh-jen) (abacavir sulfate) Tablets and Oral Solution
Generic name: abacavir (uh-BACK-ah-veer) sulfate tablets and oral solution
Read the Medication Guide that comes with Ziagen before you start taking it and each time you get a refill because there may be new information. This information does not take the place of talking to your doctor about your medical condition or your treatment. Be sure to carry your Ziagen Warning Card with you at all times.
What is the most important information I should know about Ziagen?
Serious Allergic Reaction to Abacavir. Ziagen contains abacavir (also contained in EpzicomTM and Trizivir®). Patients taking Ziagen may have a serious allergic reaction (hypersensitivity reaction) that can cause death. If you get a symptom from 2 or more of the following groups while taking Ziagen, stop taking Ziagen and call your doctor right away.
-
- Symptom(s)
- Group 1: Fever
- Group 2: Rash
- Group 3: Nausea, vomiting, diarrhea, abdominal (stomach area) pain
- Group 4: Generally ill feeling, extreme tiredness, or achiness
- Group 5: Shortness of breath, cough, sore throat
If you stop treatment with Ziagen because of this serious reaction, NEVER take Ziagen (abacavir) again. If you take Ziagen or any other abacavir-containing medicine again after you have had an allergic reaction, WITHIN HOURS you may get life-threatening symptoms that may include very low blood pressure or death.
If you stop Ziagen for any other reason, even for a few days and you are not allergic to Ziagen, talk with your doctor before taking it again. Taking Ziagen again can cause a serious allergic or life-threatening reaction, even if you never had an allergic reaction to it before. If your doctor tells you that you can take Ziagen again, start taking it when you are around medical help or people who can call a doctor if you need one.
Lactic Acidosis. Some HIV medicines, including Ziagen, can cause a rare but serious condition called lactic acidosis with liver enlargement (hepatomegaly). Nausea and tiredness that donít get better may be symptoms of lactic acidosis. In some cases this condition can cause death. Women, overweight people, and people who have taken HIV medicines like Ziagen for a long time have a higher chance of getting lactic acidosis and liver enlargement. Lactic acidosis is a medical emergency and must be treated in the hospital.
Ziagen can have other serious side effects. Be sure to read the section below entitled "What are the possible side effects of Ziagen?"
What is Ziagen?
Ziagen is a prescription medicine used to treat HIV infection. Ziagen is taken by mouth as a tablet or a strawberry-banana-flavored liquid. Ziagen is a medicine called a nucleoside analogue reverse transcriptase inhibitor (NRTI). Ziagen is always used with other anti-HIV medicines. When used in combination with these other medicines, Ziagen helps lower the amount of HIV found in your blood. This helps to keep your immune system as healthy as possible so that it can help fight infection.
Different combinations of medicines are used to treat HIV infection. You and your doctor should discuss which combination of medicines is best for you.
- Ziagen does not cure HIV infection or AIDS. We do not know if Ziagen will help you live longer or have fewer of the medical problems that people get with HIV or AIDS. It is very important that you see your doctor regularly while you are taking Ziagen.
- Ziagen does not lower the risk of passing HIV to other people through sexual contact, sharing needles, or being exposed to your blood. For your health and the health of others, it is important to always practice safe sex by using a latex or polyurethane condom or other barrier method to lower the chance of sexual contact with semen, vaginal secretions, or blood. Never use or share dirty needles.
Ziagen has not been studied in children under 3 months of age or in adults over 65 years of age.
Who should not take Ziagen?
Do not take Ziagen if you:
- have ever had a serious allergic reaction (a hypersensitivity reaction) to Ziagen or any other medicine that has abacavir as one of its ingredients (Epzicom and Trizivir). See the end of this Medication Guide for a complete list of ingredients in Ziagen. If you have had such a reaction, return all of your unused Ziagen to your doctor or pharmacist.
- have a liver that does not function properly.
Before starting Ziagen, tell your doctor about all your medical conditions, including if you:
- are pregnant or planning to become pregnant. We do not know if Ziagen will harm your unborn child. You and your doctor will need to decide if Ziagen is right for you. If you use Ziagen while you are pregnant, talk to your doctor about how you can be on the Antiviral Pregnancy Registry for Ziagen.
- are breastfeeding. We do not know if Ziagen can be passed to your baby in your breast milk and whether it could harm your baby. Also, mothers with HIV should not breastfeed because HIV can be passed to the baby in the breast milk.
Tell your doctor about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements. Especially tell your doctor if you take:
- methadone
- Epzicom (abacavir sulfate and lamivudine) and Trizivir (abacavir sulfate, lamivudine, and zidovudine)..
How should I take Ziagen?
- Take Ziagen by mouth exactly as your doctor prescribes it. Your doctor will tell you the right dose to take. The usual doses are 1 tablet twice a day or 2 tablets once a day. Do not skip doses.
- You can take Ziagen with or without food.
- If you miss a dose of Ziagen, take the missed dose right away. Then, take the next dose at the usual time.
- Do not let your Ziagen run out.
- Starting Ziagen again can cause a serious allergic or life-threatening reaction, even if you never had an allergic reaction to it before.before. If you run out of Ziagen even for a few days, you must ask your doctor if you can start Ziagen again. If your doctor tells you that you can take Ziagen again, start taking it when you are around medical help or people who can call a doctor if you need one.
- If you stop your anti-HIV drugs, even for a short time, the amount of virus in your blood may increase and the virus may become harder to treat.
- If you take too much Ziagen, call your doctor or poison control center right away.
What should I avoid while taking Ziagen?
- Do not take Epzicom (abacavir sulfate and lamivudine) or Trizivir (abacavir sulfate, lamivudine, and zidovudine) while taking Ziagen. Some of these medicines are already in Ziagen.
- Do not share needles or other injection equipment.
- Do not share personal items that can have blood or body fluids on them, like toothbrushes and razor blades.
- Do not have any kind of sex without protection. Always practice safe sex by using a latex or polyurethane condom or other barrier method to lower the chance of sexual contact with semen, vaginal secretions, or blood.
- Do not breastfeed. We do not know if Ziagen can be passed to your baby in your breast milk and whether it could harm your baby. Also, mothers with HIV should not breastfeed because HIV can be passed to the baby in the breast milk.
What are the possible side effects of Ziagen?
Ziagen can cause the following serious side effects:- Serious allergic reaction that can cause death.
- Lactic acidosis with liver enlargement (hepatomegaly) that can cause death. (See "What is the most important information I should know about Ziagen?" at the beginning of this Medication Guide.)
- Changes in body fat. These changes have happened in patients taking antiretroviral medicines like Ziagen. The changes may include an increased amount of fat in the upper back and neck (.buffalo hump.), breast, and around the back, chest, and stomach area. Loss of fat from the legs, arms, and face may also happen. The cause and long-term health effects of these conditions are not known.
The most common side effects of Ziagen include nausea, vomiting, tiredness, headache, diarrhea, trouble sleeping, fever and chills, and loss of appetite. Most of these side effects did not cause people to stop taking Ziagen.
This list of side effects is not complete. Ask your doctor or pharmacist for more information.
How should I store Ziagen?
- Store Ziagen at room temperature, between 68° to 77°F (20° to 25°C). Do not freeze Ziagen.
- Return your unused Ziagen to your doctor or pharmacist for proper disposal.
- Keep Ziagen and all medicines out of the reach of children.
General information for safe and effective use of Ziagen
Medicines are sometimes prescribed for conditions that are not mentioned in Medication Guides. Do not use Ziagen for a condition for which it was not prescribed. Do not give Ziagen to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about Ziagen. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for the information that is written for healthcare professionals or call 1-888-825-5249.
What are the ingredients in Ziagen?
Tablets: Each tablet contains abacavir sulfate equivalent to 300 mg of abacavir as active ingredient and the following inactive ingredients: colloidal silicon dioxide, magnesium stearate, microcrystalline cellulose, and sodium starch glycolate. The film-coating is made of hypromellose, polysorbate 80, synthetic yellow iron oxide, titanium dioxide, and triacetin.
Oral Solution: Each milliliter (1 mL) of Ziagen Oral Solution contains abacavir sulfate equivalent to 20 mg of abacavir (i.e., 20 mg/mL) as active ingredient and the following inactive ingredients: artificial strawberry and banana flavors, citric acid (anhydrous), methylparaben and propylparaben (added as preservatives), propylene glycol, saccharin sodium, sodium citrate (dihydrate), sorbitol solution, and water.