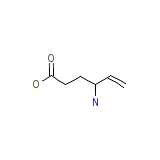
4-Aminohexenoic acid




4-Aminohexenoic acid Brand names, 4-Aminohexenoic acid Analogs
4-Aminohexenoic acid Brand Names Mixture
- No information avaliable
4-Aminohexenoic acid Chemical_Formula
C6H11NO2
4-Aminohexenoic acid RX_link
No information avaliable
4-Aminohexenoic acid fda sheet
4-Aminohexenoic acid msds (material safety sheet)
4-Aminohexenoic acid Synthesis Reference
No information avaliable
4-Aminohexenoic acid Molecular Weight
129.157 g/mol
4-Aminohexenoic acid Melting Point
No information avaliable
4-Aminohexenoic acid H2O Solubility
55.1 mg/mL
4-Aminohexenoic acid State
Solid
4-Aminohexenoic acid LogP
0.056
4-Aminohexenoic acid Dosage Forms
Powder (500 mg sachets); Tablet (500 mg)
4-Aminohexenoic acid Indication
For use as an adjunctive treatment (with other drugs) in treatment resistant epilepsy, complex partial seizures, secondary generalized seizures, and for monotherapy use in infantile spasms in West syndrome.
4-Aminohexenoic acid Pharmacology
Vigabatrin, is an anticonvulsant chemically unrelated to other anticonvulsants. Vigabatrin inhibits the catabolism of GABA. It is an analog of GABA, but it is not a receptor agonist.
4-Aminohexenoic acid Absorption
Rapidly absorbed following oral administration. Food may slightly decrease the rate, but not the extent, of absorption.
4-Aminohexenoic acid side effects and Toxicity
No information avaliable
4-Aminohexenoic acid Patient Information
This medicine may reduce your ability to drive or operate machinery safely. Do not drive or operate machinery until you know how this medicine affects you and you are sure it won't affect your performance. Avoid stopping this medicine suddenly. The dose of this medicine should be gradually decreased over a period of two to four weeks. After months to years of taking this medication, damage to vision may occur. Regular eye examinations should be conducted and notify your doctor if any change in vision occurs.
4-Aminohexenoic acid Organisms Affected
Humans and other mammals