3377 RP opalate




3377 RP opalate Brand names, 3377 RP opalate Analogs
- 3377 RP opalate
- Amokin
- Aralen
- Aralen HCl
- Arechin
- Arthrochin
- Artrichin
- Avlochlor
- Avloclor
- Bemaco
- Bemaphate
- Bemasulph
- Benaquin
- Bipiquin
- Capquin
- Chemochin
- Chingamin
- Chloraquine
- Chlorochin
- Chlorochine
- Chloroquina
- Chloroquine (VAN)
- Chloroquine Phosphate
- Chloroquinium
- Chlorquin
- Cidanchin
- Clorochina
- Cocartrit
- Delagil
- Dichinalex
- Elestol
- Gontochin
- Heliopar
- Hydroxychloroquine Sulfate
- Imagon
- Iroquine
- Klorokin
- Lapaquin
- Malaquin
- Malaren
- Malarex
- Mesylith
- Neochin
- Nivachine
- Nivaquine
- Nivaquine B
- Nivaquine [as sulfate]
- Pfizerquine
- Plaquenil
- Quinachlor
- Quinagamin
- Quinagamine
- Quinercyl
- Quingamine
- Quinilon
- Quinoscan
- Resochen
- Resochin
- Resoquina
- Resoquine
- Reumachlor
- Reumaquin
- Roquine
- Sanoquin
- Silbesan
- Siragan
- Solprina
- Sopaquin
- Tanakan
- Tresochin
- Trochin
3377 RP opalate Brand Names Mixture
- No information avaliable
3377 RP opalate Chemical_Formula
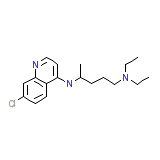
C18H26ClN3
3377 RP opalate RX_link
http://www.rxlist.com/cgi/generic2/hquine2.htm
3377 RP opalate fda sheet
3377 RP opalate msds (material safety sheet)
3377 RP opalate Synthesis Reference
No information avaliable
3377 RP opalate Molecular Weight
319.872 g/mol
3377 RP opalate Melting Point
289 oC
3377 RP opalate H2O Solubility
10.6 mg/L
3377 RP opalate State
Solid
3377 RP opalate LogP
4.474
3377 RP opalate Dosage Forms
Tablet
3377 RP opalate Indication
For the suppressive treatment and for acute attacks of malaria due to P. vivax, P.malariae, P. ovale, and susceptible strains of P. falciparum, Second-line agent in treatment of Rheumatoid Arthritis
3377 RP opalate Pharmacology
Chloroquine is the prototype anti malarial drug, most widely used to treat all types of malaria except for disease caused by chloroquine resistant Plasmodium falciparum. It is highly effective against erythrocytic forms of Plasmodium vivax, Plasmodium ovale and Plasmodium malariae, sensitive strains of Plasmodium falciparum and gametocytes of Plasmodium vivax. Being alkaline, the drug reaches high concentration within the food vacuoles of the parasite and raises its pH. It is found to induce rapid clumping of the pigment. Chloroquine inhibits the parasitic enzyme heme polymerase that converts the toxic heme into non-toxic hemazoin, thereby resulting in the accumulation of toxic heme within the parasite. It may also interfere with the biosynthesis of nucleic acids.
3377 RP opalate Absorption
Completely absorbed from gastrointestinal tract
3377 RP opalate side effects and Toxicity
No information avaliable
3377 RP opalate Patient Information
PATIENT INFORMATION
Complete blood cell counts should be made periodically if patients are given prolonged therapy. If any severe blood disorder appears which is not attributable to the disease under treatment, discontinuance of the drug should be considered. The drug should be administered with caution to patients having G-6-PD (glucose-6 phosphate dehydrogenase) deficiency.
In patients with preexisting auditory damage, chloroquine should be administered with caution. In case of any defects in hearing, chloroquine should be immediately discontinued, and the patient closely observed.
Since this drug is known to concentrate in the liver, it should be used with caution in patients with hepatic disease or alcoholism or in conjunction with known hepatotoxic drugs.
Patients with history of epilepsy should be advised about the risk of chloroquine provoking seizures.
Because of the potential for serious adverse reactions in nursing infants from chloroquine, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the potential clinical benefit of the drug to the mother.
Irreversible retinal damage has been observed in some patients who had received long-term or high-dosage 4-aminoquinoline therapy. Retinopathy has been reported to be dose related.
Follow Rxlist link and drugs.com link for detailed patient information.
Complete blood cell counts should be made periodically if patients are given prolonged therapy. If any severe blood disorder appears which is not attributable to the disease under treatment, discontinuance of the drug should be considered. The drug should be administered with caution to patients having G-6-PD (glucose-6 phosphate dehydrogenase) deficiency.
In patients with preexisting auditory damage, chloroquine should be administered with caution. In case of any defects in hearing, chloroquine should be immediately discontinued, and the patient closely observed.
Since this drug is known to concentrate in the liver, it should be used with caution in patients with hepatic disease or alcoholism or in conjunction with known hepatotoxic drugs.
Patients with history of epilepsy should be advised about the risk of chloroquine provoking seizures.
Because of the potential for serious adverse reactions in nursing infants from chloroquine, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the potential clinical benefit of the drug to the mother.
Irreversible retinal damage has been observed in some patients who had received long-term or high-dosage 4-aminoquinoline therapy. Retinopathy has been reported to be dose related.
Follow Rxlist link and drugs.com link for detailed patient information.
3377 RP opalate Organisms Affected
Plasmodium